Identify the Appropriate Arrow in the Equation Shown.
Match the numbers in the left column to the appropriate blanks in the chemical equations on the right. Identify the transformation type for the following equation set.
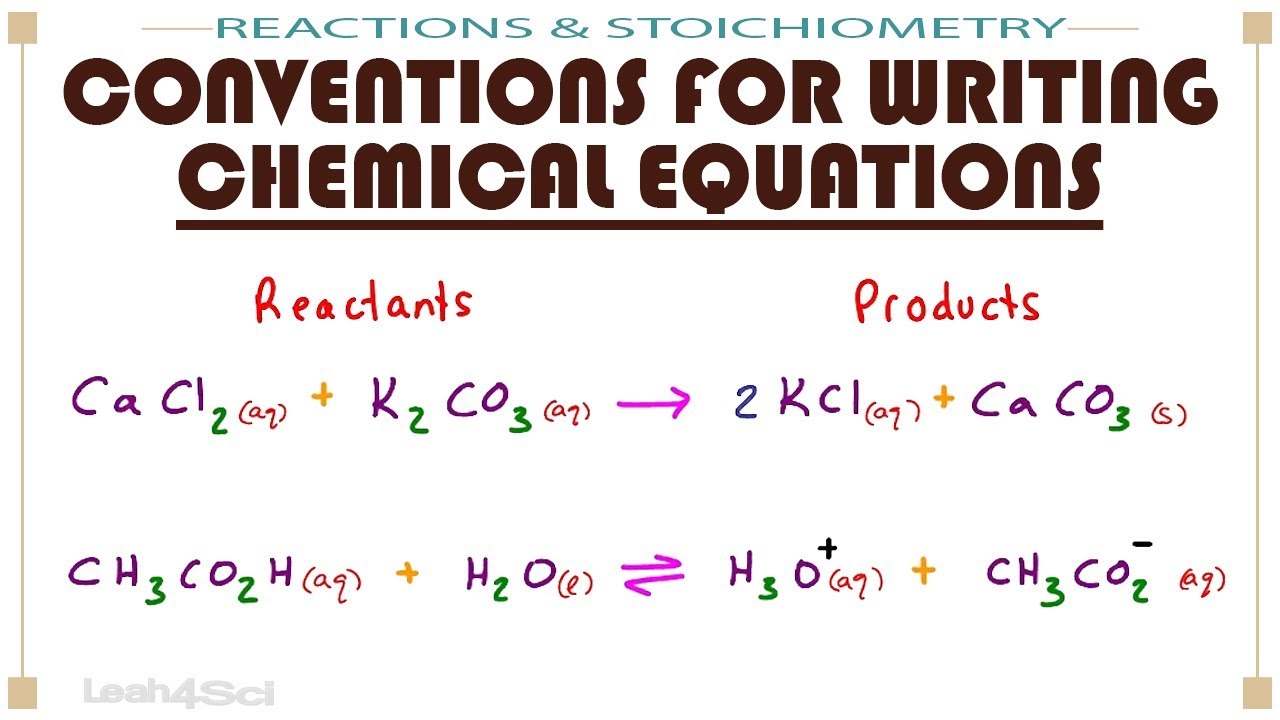
Conventions For Writing Chemical Equations Arrows Phases Coefficients And More Youtube
Write the equation in slopeintercept form.
. Write the standard form of the equation and the general form of the equation of each circle of radius r and center hk. CH 4 g 2 O 2 g CO 2 g 2H 2 O g The reactants are written on the left side of the arrow while the products are written on the right side of the arrow. Solutions for Chapter 1 Problem 71P.
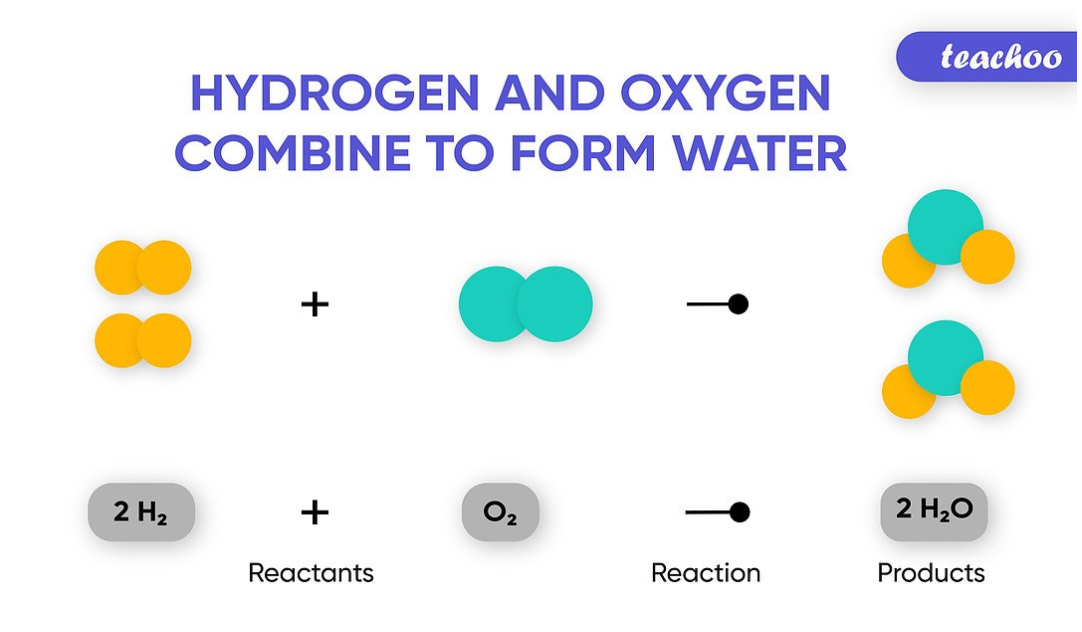
The reaction between methane and oxygen to yield carbon dioxide and water shown at bottom may be represented by a chemical equation using formulas top. To simplify the differential equation lets divide out the mass m m. The labels a b c and d serve as references and have no other significance.
Ionic charges are not yet supported and will be ignored. The curved arrow with a single barb on the arrowhead denotes the path of an electron in a reaction. N n minus one equals fourteen.
To do this right-click on an atom and choose Atom properties. The chemical equation representing this process is provided in the upper half of Figure 1 with space-filling molecular models shown in the lower half of the figure. A-island- fused b-penninsulabay- simplified c-island-fused.
The sum of three and five is equal to eight. Here are some examples of equations. E 1101 - 22X - 78Y N 942.
Balance the given equation using half. Calculate the resampled value at the point shown given the raster neighborhood and using a bilinear interpolator 18 box. Following the curved arrows complete each equation to show the products formed.
A complete ionic equation is a chemical equation in which the dissolved ionic compounds are written as separated ions. The balanced equation will appear above. Hk -21 arrow_forward.
An acid or base will ionize or dissociate completely in water whereas an acid or base produces relatively few ions in water. Therefore they are termed as spectator ions. Select the six atoms two for each bond that participate in covalent bonds in the reactants that will break according to the curved electron arrows.
Make certain the chemical equation is complete before submitting your answer. They remain unchanged throughout the equation. For each of the following write the chemical equation with appropriate equilibrium arrows as shown in Table 5.
At this point our only option for sketching a parametric curve is to pick values of t t plug them into the parametric equations and then plot the points. Hence the reactants and products in the given equation are CH 4 O 2 and CO 2 H 2 O respectively. Left as well as right side of the equation.
6742 6 7 42. Find an equation of a line that contains the points and. Dv dt g γv m 1 1 d v d t g γ v m.
Curved arrows are usually shown at individual atoms in a skeletal structure to show where the electron is moved from to in the product molecule. Identify the arrow in the following equation. X t2 t y 2t1 x t 2 t y 2 t 1.
A The Haber process is used to manufacture ammonia fertilizer from hydrogen and nitrogen gases. 358 3 5 8. The electron moves from the tail to the head.
Solved Identify the arrow in the following equation. The circuit consists of a voltage source and three external load resistors. N114 n 1 14.
Since we have two points we will find an equation of the line using the pointslope form. Identify the appropriate group of atoms by selecting each atom individually on the canvas and assigning them a map number of 1 until all atoms are mapped. Solubility rules are very useful in.
The first step will be to find the slope. When chemicals in solution react the proper way of writing the chemical formulas of the dissolved ionic compounds is in terms of the dissociated ions not the complete ionic formula. Identify the element oxidized element reduced oxidizing agent and reducing agent.
Kirchhoffs loop rule states that the algebraic sum of the voltage differences is equal to zero. K aq OH aq H aq NO 3 aq K aq NO 3 aq H 2 O l From the above equation it can be observed that Kaq and NO3aq are present on both. Use uppercase for the first character in the element and lowercase for the second character.
All of the substances shown in the following acid-base reactions are found in Table 18 and the equilibrium lies to the right in each case. Identify the arrow in the following equation. Example 1 Sketch the parametric curve for the following set of parametric equations.
The double arrows in the balanced equation given indicate that the reaction of a weak acid with water is. Indicated by the black arrow on the left. The product of six and seven is equal to forty-two.
To balance a chemical equation enter an equation of a chemical reaction and press the Balance button. Br Br -. Balance the chemical equation by indicating the number of each species in the appropriate blanks.
Fe Au Co Br C O N F. Identify the acid base conjugate acid and conjugate base. This then is a first order linear differential equation that when solved will give the velocity v v in ms of a falling object of mass m m that has both gravity and air resistance acting upon it.
For this exercise indicate coefficients of 1 explicitly.
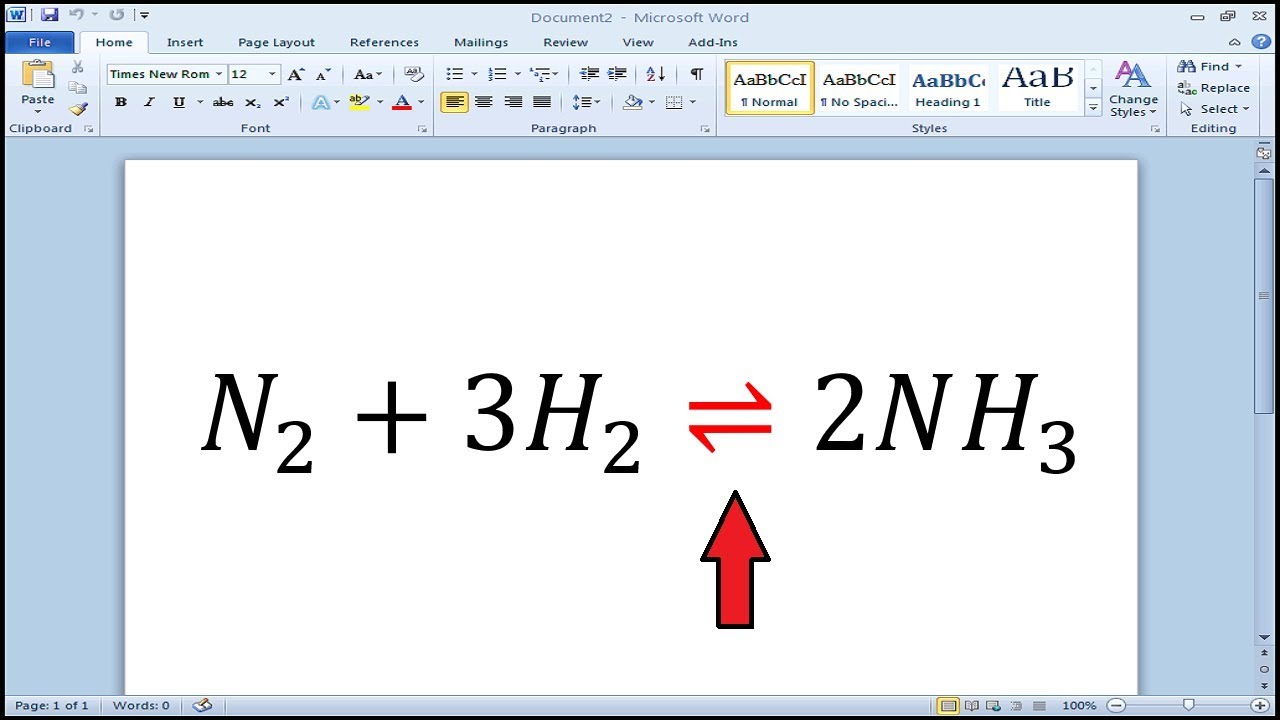
How To Write Reversible Reaction Arrow Symbol In Word Youtube
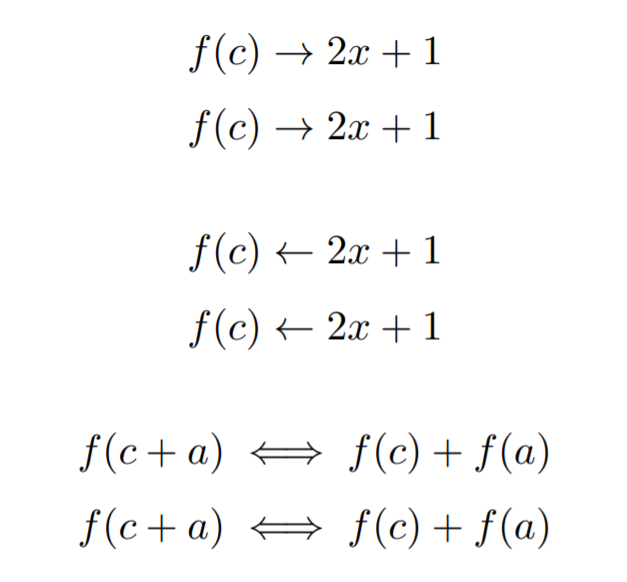
Arrow Types In Latex A Complete List Latex Tutorial Com

What Does An Arrow Mean In A Chemical Equation Study Guide Inspirit
No comments for "Identify the Appropriate Arrow in the Equation Shown."
Post a Comment